Growing emphasis on small molecular diagnostics is prompting key pharmaceutical giants to initiate clinical trials furthering development of investigational drug candidates
Dubai, United Arab Emirates - March 17, 2021 /MarketersMedia/ —
Future Market Insights, Dubai: ESOMAR-certified Future Market Insights’ recent APOL-1 mediated kidney disease market report forecasts a healthy growth outlook through 2021 and beyond. As of 2020, the market was valued at US$ 3 billion, majorly bolstered by ongoing active clinical pipeline projects to discover potentially new drug candidates. Future projections indicate a positive forecast, with an anticipated CAGR exceeding 4% through 2031.
According to the National Kidney Foundation, as of 2015, 10% of the global population was affected by chronic kidney disease. As of 2019, over 750 million people were impacted. Consequently, research and development spending to address this burden has inclined in recent years. Between 2016 and 2017, global R&D spending amounted to nearly 4%, costing around US$ 97.2 billion. Such promising figures are opening up future investment opportunities by prospective pharmaceutical giants.
Recently, in February 2021, British pharmaceutical giant AstraZeneca received marketing authorization to advocate its anti-diabetic drug dapagliflozin for treating patients in India suffering from chronic kidney disease. The company has already made significant strides, negotiating an over US$ 300 million licensing agreement with Ionis Pharmaceuticals for two pipeline antisense therapies in 2018. The candidate under investigation is the AZD2373 for treating the original cause of APOL-1 CKDs.
“As the global chronic kidney diseases burden increases, R&D projects to accelerate potential drug candidate availability are rising, generating massive revenue pools across key markets, heightening growth prospects,” says the FMI analyst.
Request a report sample with 95 pages to gain comprehensive insights at https://www.futuremarketinsights.com/reports/sample/rep-gb-12115
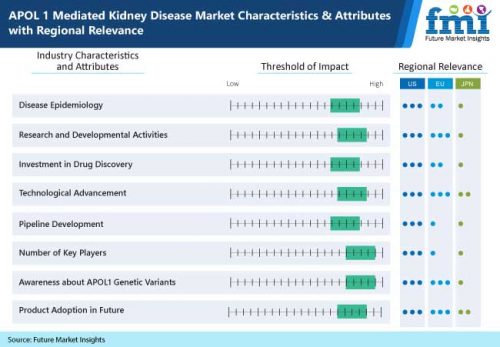
Key Takeaways from FMI’s APOL-1 Mediated Kidney Disease Market Study
By indication, focal segmental glomerulosclerosis (FSGS) to remain the primary therapeutic domain
US to experience elevated growth prospects amid rising frequencies of ongoing multiple stage small molecule inhibitor clinical trials
Foray for investigating effective diagnosis to prevent end stage renal disease (ESRD) spurring APOL-1 mediated kidney disease treatment in the UK
Germany & France concentrating on providing affordable nephrology care, thus widening growth scope
High prevalence of diabetic neuropathy is compelling patients to seek treatment for kidney disorders, broadening growth prospects across India
APOL-1 Mediated Kidney Disease Market- Prominent Drivers
Preference for small molecule genetics is catapulting growth prospects for APOL-1 mediated kidney disease treatment
Highly consolidated landscape is paving way for future penetration by new players, broadening research capabilities
Broader budgetary allocation to facilitate increased research is expected to propel the market forward
APOL-1 Mediated Kidney Disease Market- Key Restraints
Rapid genetic mutations may render it difficult to develop a standardized treatment approach, thereby hindering growth prospects
Limited awareness regarding the onset of chronic kidney disorders leads to quick progression towards ESRD, rendering effective treatment difficult
Discover more about the APOL-1 mediated kidney disease market with 15 figures and 4 data tables, along with the table of contents. You will also find detailed market segmentation on https://www.futuremarketinsights.com/reports/apol-1-mediated-kidney-disease-market
Competitive Landscape
Prominent players featured in FMI’s APOL-1 mediated kidney disease market include Vertex Pharmaceuticals Incorporated, Ionis Pharmaceuticals Inc. (AstraZeneca), Retrophin Inc., ChemoCentryx Inc., Variant Pharmaceuticals Inc., GlaxoSmithKline Plc., Novartis AG and Teva Pharmaceuticals Ltd. among others.
Majority of these players are emphasizing on developing small molecule inhibitors to prevent the onset of the disease. In October 2020, Vertex Pharmaceuticals announced it was working on the VX-147 small molecule inhibitor for APOL-1 mediated focal segmental glomerulosclerosis (FSGS), which entered its Phase 2 clinical trial.
Likewise, in May 2020, ChemoCentryx Inc. announced topline data from a 46 patient Phase II dose-ranging trial for primary FSGS titled LUMINA-1. The trial tested CCX140, an orally administered selective inhibitor of the CCR2 chemokine receptor, the main trigger for the disease.
More Insights on FMI’s APOL-1 Mediated Kidney Disease Market
Future Market Insights brings the comprehensive research report on forecasted revenue growth at global, regional, and country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2016-2020 and forecast from 2021-2031. The global APOL1 mediated kidney disease market is segmented in detail to cover every aspect of the market and present a complete market intelligence approach to the reader. The study provides compelling insights on APOL1 mediated kidney disease market on basis of indication (chronic kidney disease and end stage kidney disease) across North America, Europe and Middle East and Africa.
Explore FMI’s Extensive Coverage on the Healthcare Domain
Single Dose Radiotherapy Services Market: In its new study, ESOMAR-certified market research and consulting firm Future Market Insights (FMI) offers insights about key factors driving demand for single-dose radiotherapy. The report tracks the global sales of single-dose radiotherapy in 20+ high-growth markets, along with analyzing the impact COVID-19 has had on radiotherapy services in general, and single-dose radiotherapy services in particular.
Cancer Tissue Diagnostics Market: The global cancer tissue diagnostics market report by FMI sheds light on the important growth dynamics expected to prevail across the 2021-2031 assessment period. Statistics of key segments have been provided across prominent geographies, along with a detailed mapping of the global competitive landscape, rendering this insight a highly effectual one.
Wound Debridement Devices Market: In its new report, the Future Market Insights (FMI) offers an exhaustive overview of the global wound debridement products market with focus on the key market dynamics, including drivers, trends, opportunities, restraints, and detailed information about the global advanced wound debridement products market structure. The market study presents exclusive information about how the market will grow during the forecast period of 2021 to 2031.
About Future Market Insights (FMI)
Future Market Insights (FMI) is a leading provider of market intelligence and consulting services, serving clients in over 150 countries. FMI is headquartered in Dubai, and has delivery centers in the UK, U.S. and India. FMI's latest market research reports and industry analysis help businesses navigate challenges and make critical decisions with confidence and clarity amidst breakneck competition. Our customized and syndicated market research reports deliver actionable insights that drive sustainable growth. A team of expert-led analysts at FMI continuously tracks emerging trends and events in a broad range of industries to ensure that our clients prepare for the evolving needs of their consumers.
Contact Info:
Name: Abhishek Budholiya
Email: Send Email
Organization: Future Market Insights
Website: https://www.futuremarketinsights.com/reports/apol-1-mediated-kidney-disease-market
Source: MarketersMedia
Release ID: 89001390
