
ROCKVILLE, Md. - August 31, 2018 - (Newswire.com)
At Sunascen Therapeutics, we are well aware of the long term effects from the chronic use of various pain relievers, such as non-steroidal anti-inflammatory drugs (NSAIDs).1-4 Moreover, the current Opioid Crisis has raised concerns throughout the world over current practices for pain management, and prescription drug use.5,6 As a result, with the patient and the purpose in mind, we have strategically developed a new line of OTC pain relievers called MYGRIN®, bearing the slogan “Helping you turn the face of pain into a grin”™. Along with MYGRIN® products, the patient/consumer will have access to a simplified pain rating scale, a self-assessment tool that can also be used by health professionals and caregivers, which is designed to assess or help rate an individual’s level of pain. By implementing the use of this scale along with the use of MYGRIN® products for pain relief, we are able to bring together the necessary message of efficiently treating pain by providing relief through proper selection, and minimum effective dosing in order to reduce long-term complications.
“Through this new product line, the patient/consumer will be able to achieve sufficient pain relief emanating from common causes by consuming the appropriate non-habit forming product along with a minimum but effective dose; without exceeding the maximum recommended daily dose unless directed by a doctor. By the same token, if the patient or consumer’s pain is persistent, we suggest for them to seek medical attention from a pharmacist or a physician for the most appropriate diagnosis and treatment plan. Generally speaking, it is highly beneficial for the patient/consumer to seek assistance from a pharmacist or a physician when selecting certain types of OTC pain reliving products, as it is often an individualized treatment approach; especially in light of eCommerce being commonplace. Furthermore, both active and inactive ingredients in MYGRIN® products have been carefully selected to provide the patient/consumer with the maximum benefit, and minimum potential long-term complications. Consequently, we are confident that MYGRIN® products will not only adequately treat or reduce the level of pain our consumers are experiencing, but they will also convey an everlasting message by altering the mindset of consuming more than necessary.” said Sandip J. Patel, PharmD; Founder, President & CEO of Sunascen Therapeutics LLC.
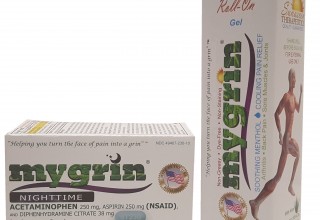
Currently Marketed MYGRIN® Products:
MYGRIN® products were strategically developed with both, patient and purpose in mind. Currently, the OTC MYGRIN® lineup consists of two products designed to alleviate pain often originating from common causes.
The first product is MYGRIN® Roll-On Gel (Menthol 3.5%), which is a specially formulated topical gel that can be used year-round in providing temporary cooling pain relief from minor aches and pains of muscles and joints associated with arthritis, backache, bruises, strains, and sprains.7-10 Along with its hands free roll on applicator, the dye free, non-greasy, and non-staining formulation is ideal for anyone 2 years of age and older. Additionally, we have carefully selected inactive ingredients such as aloe barbadensis leaf extract, angelica archangelica root extract, and panax ginseng root extract to provide additional benefits to the consumer. It should also be mentioned that MYGRIN® Roll-On Gel’s pleasantly light and vanishing scent will not have surrounding individuals questioning your health needs either.
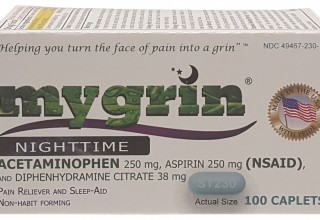
The second product is MYGRIN® Nighttime (Acetaminophen 250mg, Aspirin 250mg (NSAID), and Diphenhydramine citrate 38mg), a combination drug used for the temporary relief of occasional headaches, and minor aches and pains, with accompanying sleeplessness. MYGRIN® Nighttime contains less than the typical single dose of most NSAIDs in each caplet.11 It also contains a less than the typical single dose of Acetaminophen, a centrally acting analgesic and antipyretic, in each caplet.12 By providing a combination of two common pain relievers at a less than typical single dose, a patient/consumer will be able to help relieve their pain and likely reduce long term complications caused by the chronic use of NSAIDs, or Acetaminophen, alone. Complementary to our internal research and development activities, published clinical studies have shown that the combination of Acetaminophen and Aspirin is effective in the reduction of pain intensity for patients.13-15 Moreover, published studies have shown that acute and chronic pain causes cognitive impairment.16,17 Additionally, sleep deprivation is also known to adversely affect the brain, its cognitive function, and influence other health problems.18-21 “Insomnia is by far the most common sleep disorder in headache patients.”22 Therefore, the need for a good night’s rest is harmonious to sufficient pain relief. Lastly, the addition of Diphenhydramine citrate, a non-addictive sleep aid, in MYGRIN® Nighttime creates an excellent combination of three active ingredients to help the patient/consumer alleviate their pain, as well as helping them fall asleep.
Since 2011, Sunascen Therapeutics has remained committed to developing and promoting superior quality products designed to improve and protect the consumer’s health. We are continuously seeking various retailers and healthcare organizations interested in making MYGRIN® products available to consumers throughout the United States of America by year-end 2018. Furthermore, we anticipate more MYGRIN® products to be released in the near future.
For more information on MYGRIN®, please visit http://www.sunascen.com/Portfolio.html
About Sunascen Therapeutics LLC
Sunascen Therapeutics applies its extensively researched science in medicine, and its technical resources to develop pharmaceutical and healthcare products, which are designed to help ease suffering, and enhance quality of life. Consistent with their corporate strategy, corporate responsibility, and their consumer’s expectations, the company values the discovery and development of quality healthcare products, diagnostic tools, and medical devices for both prescription and OTC items. Moreover, it relentlessly aims to exceed the current standard for quality, safety, and effectiveness in order to provide consumers with the best possible option to manage their health needs.
Sunascen Therapeutics is continuously strengthening its product portfolio by searching for business partners interested in the development, as well as co-development, of new pharmaceutical products which fit within, and complement the corporate and product development strategy. The company builds strategic partnerships with existing, and emerging companies to develop and commercialize safe, effective, and innovative treatments for diseases which have insufficient treatment options, or medical needs that remain unmet including those of orphan diseases. Sunascen Therapeutics offers a full range of product development and support services designed to accelerate innovation and efficiently execute the approval process, new market entry, and support while maintaining full regulatory compliance. Their production, operations, and corporate governance are based in the United States of America.
For more information on Sunascen Therapeutics LLC, please visit http://www.sunascen.com
Corporate Contact:
Sandip J. Patel, PharmD
Founder, President & CEO
Sunascen Therapeutics LLC
(240) 403-2614
info@sunascen.com
FORWARD-LOOKING STATEMENTS AND CAUTIONARY NOTE
Statements in this press release include forward-looking statements regarding Sunascen Therapeutics LLC’s financial position and outlook, as well as Sunascen Therapeutics’ business. These forward-looking statements are based on current expectations, which involve assumptions, significant unforeseen uncertainties and risks, as well as potential changes in circumstances. Statements describing Sunascen Therapeutics goals, expectations, financial projections or other business related projections, intentions, beliefs, including the planned commercialization of any product, are considered forward-looking statement and shall be considered an at-risk statement. At-risk statements are subject to uncertainties, particularly those intrinsic to the discovery, development, and commercialization process for pharmaceutical and life science products, which are safe and effective for use as human therapies and in the venture of building a business around regulatory approved drugs. Furthermore, forward-looking statements discussed by Sunascen Therapeutics involve a certain degree of assumptions, which if never materialized nor proven correct, may possibly cause results to differ materially from those otherwise implied or expressed by such forward-looking statements. As a result, Sunascen Therapeutics advises the use of caution for forward-looking statements. Therefore, no assurance is implied or will be given to the outcomes stated in such forward-looking statements, and whether certain estimates will be achieved. Although forward-looking statements made by Sunascen Therapeutics emulate the good faith judgment of its management, these statements are based solely on factual information and factors currently known by Sunascen Therapeutics. The statements provided in this press release are based on the date of this release, and Sunascen Therapeutics assumes no commitment, and therefore explicitly declines any commitment to revise or update any forward-looking statements, resulting from future events or new information as time may present.
In the above press release, unless the context specifies or requires otherwise, "Sunascen Therapeutics", "Company", "we", “us”, and "our" directly refer to Sunascen Therapeutics LLC. “Sunascen”, “Sunascen Therapeutics”, and “MYGRIN®” are trademarks of Sunascen Therapeutics LLC. MYGRIN® products are distributed by Sunascen Therapeutics LLC.
References:
1 Marcum Z.A., Hanlon J.T. (2010). “Recognizing the Risks of Chronic Non-Steroidal Anti-Inflammatory Drug Use in Older Adults.” Annals of Long-Term Care, 18(9), 24–27. PMID: 21857795
2 Cryer B., Barnett M.A., Wagner J., Wilcox C.M. (2016NOV). “Overuse and Misperceptions of Non-Steroidal Anti-Inflammatory Drugs in the United States.” The American Journal of the Medical Sciences. 352(5):472-480. PMID: 27865294
3 Kasarla, M. (2018JUL18) “The Overuse of NSAIDs.” Physician's Weekly. [ONLINE] Available at: https://www.physiciansweekly.com/the-overuse-of-nsaids/ Retrieved [2018AUG01]
4 Vandenbussche N., Laterza D., Lisicki M., Lloyd J., et.al. (2018JUL13) “Medication-overuse headache: a widely recognized entity amidst ongoing debate” The Journal of Headache and Pain. 19(1): 50. PMID: 30003412
5 “The Opioid Crisis.” (2018) The White House, The United States Government. [ONLINE] Available at: https://www.whitehouse.gov/opioids/ Retrieved [2018AUG11]
6 “CDC Guideline for Prescribing Opioids for Chronic Pain.” (2017AUG29). Centers for Disease Control and Prevention, U.S. Department of Health & Human Services. [ONLINE] Available at: https://www.cdc.gov/drugoverdose/prescribing/guideline.html Retrieved [2018AUG01].
7 Fallon M.T., Storey D.J., Krishan A., Weir C.J. et.al. (2015SEP). “Cancer treatment-related neuropathic pain: proof of concept study with menthol--a TRPM8 agonist.” Supportive Care in Cancer. 23(9):2769-77 PMID: 25680765
8 Gaudioso C., Hao J., Martin-Eauclaire M.F., Gabriac M., et.al. (2012FEB). “Menthol pain relief through cumulative inactivation of voltage-gated sodium channels.” Pain. 153(2):473-84 PMID: 22172548
9 Topp R, Brosky J.A. Jr, Pieschel D. (2013APR-JUN). “The effect of either topical menthol or a placebo on functioning and knee pain among patients with knee OA.” Journal of Geriatric Physical Therapy. 36(2):92-9 PMID: 22976810
10 Tokunaga T., Sugawara H., Tadano C., Muro M. (2017JUN). “Effect of stimulation of cold receptors with menthol on EMG activity of quadriceps muscle during low load contraction” Somatosensory & Motor Research. 34(2):85-91 PMID: 28325123
11 “Bayer Aspirin Regimen-325mg” (2017MAY03). National Institutes of Health, U.S. National Library of Medicine, Health & Human Services. [ONLINE] Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=0c6ae05f-7634-34d1-e054-00144ff88e88 Retrieved [2018AUG01].
12 “TYLENOL REGULAR STRENGTH” (2017NOV04). National Institutes of Health, U.S. National Library of Medicine, Health & Human Services. [ONLINE] Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=1622f694-4d63-4c56-8737-fae31f0ecfb7 Retrieved [2018AUG01].
13 Diener HC, Peil H, Aicher B. (2011OCT). “The efficacy and tolerability of a fixed combination of acetylsalicylic acid, paracetamol, and caffeine in patients with severe headache: a post-hoc subgroup analysis from a multicentre, randomized, double-blind, single-dose, placebo-controlled parallel group study.” Cephalalgia. 31(14):1466-76. PMID: 21908446
14 Goldstein J., Silberstein S.D., Saper J.R., Ryan R.E. Jr, et.al. (2006MAR). “Acetaminophen, aspirin, and caffeine in combination versus ibuprofen for acute migraine: results from a multicenter, double blind, randomized, parallel-group, single-dose, placebo-controlled study.” Headache. 46(3):444-53. PMID: 16618262
15 Diener HC, Pfaffenrath V, Pageler L, Peil H, et.al (2005OCT). “The fixed combination of acetylsalicylic acid, paracetamol and caffeine is more effective than single substances and dual combination for the treatment of headache: a multicentre, randomized, double-blind, single-dose, placebo-controlled parallel group study.” Cephalalgia. 25(10):776-87. PMID: 16162254
16 Hart R.P., Wade J.B., Martelli M.F. (2003APR) “Cognitive impairment in patients with chronic pain: the significance of stress.” Current Pain and Medical Reports. 7(2):116-26. PMID: 12628053
17 Van der Leeuw G., Ayers E., Leveille S.G., Blankenstein A.H. et.al. (2018JUL09) “The effect of pain on major cognitive impairment in older adults.” The Journal of Pain: Official Journal of the American Pain Society. Pii: S1526-5900(18)30328-6. PMID: 30004021
18 Alhola P., Polo-Kantola P. (2007) “Sleep deprivation: Impact on cognitive performance.” Neuropsychiatric disease and treatment. 3(5):553-67. PMID: 19300585
19 Kong D., Liu R., Song L., Zheng J. et.al (2018JUL16) “Altered Long- and Short-Range Functional Connectivity Density in Healthy Subjects after Sleep Deprivations.” Frontiers in Neurology. 9:546 PMID: 30061857
20 Lowe C.J., Safati A., Hall P.A. (2017SEP) “The neurocognitive consequences of sleep restriction: A meta-analytic review.” Neuroscience and Biobehavioral Reviews. 80:586-604 PMID: 28757454
21 Vgontzas A.N., Mastorakos G., Bixler E.O., Kales A., et.al. (1999AUG) “Sleep deprivation effects on the activity of the hypothalamic-pituitary-adrenal and growth axes: potential clinical implications.” Clinical Endocrinology. 51(2):205-15 PMID: 10468992
22 Rains J.C. (2018AUG10) “Sleep and Migraine: Assessment and Treatment of Comorbid Sleep Disorders.” Headache. PMID: 30095163
Related Files
SunascenTherapeutics_PressRelease08122018.pdf
Related Images

Press Release Service by Newswire.com
Original Source: Sunascen Therapeutics LLC Announces a New Line of OTC Pain Relievers in Addition to a Simplified Pain Rating Scale
