AbbVie (NYSE: ABBV) shares have been under pressure for the last 2 years as fears of the patent cliff gripped the market. The fear of rapidly declining Humira sales was not offset by hope driven by new products such as Skyrizi and Rinvoq. The takeaway for today is that Humira sales may already be stabilizing, and Skrizi and Rinvoq are doing a fair share of heaving lifting for the business.
AbbVie (NYSE: ABBV) shares have been under pressure for the last 2 years as fears of the patent cliff gripped the market. The fear of rapidly declining Humira sales was not offset by hope driven by new products such as Skyrizi and Rinvoq. The takeaway for today is that Humira sales may already be stabilizing, and Skrizi and Rinvoq are doing a fair share of heaving lifting for the business.
What this means is that the Q2 results were better than expected because of the relative strengths, and guidance was lifted, sparking a rally in the stock that the analysts support. The market may not rise to a new all-time high, but this high-yielding pharma company stock is ready to try it.
AbbVie Heals An Ailing Market
AbbVie had a solid quarter despite a 25% YOY decline in its leading product, Humira. The company brought in $13.87 billion in net revenue, a decline of 4.9% compared to last year. The good news is that revenue beat the consensus by $0.350 billion or 260 basis points on relative strength in Humira and robust growth in the next 2 leading products, Skyrizi and Rinvoq.
Sales of Humira are down YOY but up 14% on strength in the US. This is mildly surprising given the number of biosimilars on the market but a sign of underlying strength.
Sales of Skyrizi and Rinvoq both jumped more than 50%, with Skyrizi topping $1.88 billion and Rinvoq quickly approaching the blockbuster threshold. On a segment basis, Immunology, including Humira, fell by 5.5%, while Hematology Oncology fell by 10.4%. Aesthetics and Neurology, including Botox therapies, grew by 1% and 13.6%.
The margin news is mixed with margin contracting compared to last year but less than expected. The adjusted EPS fell by 13.6% compared to the top-line -4.9% but outpaced the consensus by a dime. That’s worth 350 basis points and plays into the guidance. Management raised guidance for the year due to the Q2 performance and expects earnings above the Marketbeat.com consensus target.
The new low-end of the range is $10.90 compared to the previous high of $10.97 and the consensus of $10.89. Based on the business momentum, this guidance could be increased for a 3rd time when the next earnings report is released.
Analysts Support High-Yielding AbbVie
The analyst activity in AbbVie stock is mixed this year, but the details are bullish. 16 analysts are rating the stock at Hold, the Hold rating is down from last year’s Moderate Buy, but the price target is above the price action and trending higher.
There were some price target reductions in April and another in July, but those are offset by 2 initiated coverages in July that have the stock pegged at Buy. Because the Q2 results and guidance align with a rising price target, investors should expect to see the positive trend continue.
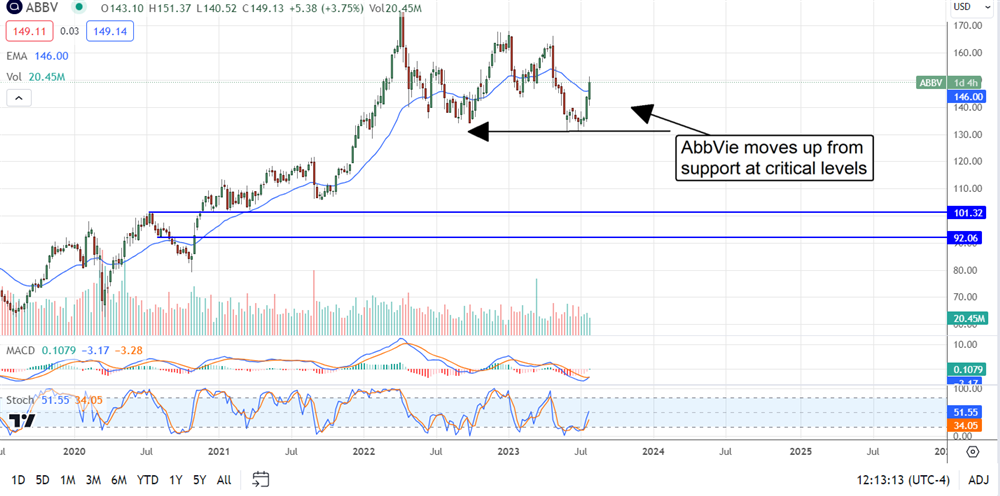
As it is, the consensus price target is about 9% above the price action and just below a critical resistance point. That point is near $165, marked by a top formed in early 2022.
AbbVie’s High Yield Helps Support The Price Action
As mixed as the outlook for Humira and AbbVie have been over the last 2 years, the stock has always found support because the dividend outlook was reliable. The stock pays about 4%, with shares at $150 with a payout ratio near 55%. That is on the high side but a manageable payout given the balance sheet and cash flow.
The balance sheet is solid, although there is a bit of debt on it. Investors may not want to expect robust dividend increases, but dividend increases should continue annually for the foreseeable future.
Shares of AbbVie are up more than 5% on the Q2 release. The market is forming another strong green candle on the weekly and daily charts that suggests a continuation of the trend will unfold.
The market may pause along the way in this scenario but is heading up to the $165 level. If it can get above that, it may trigger another wave of buying.