The biopharmaceutical landscape is continuously evolving, with the convergence of innovation and tradition unlocking novel therapeutic opportunities. Curanex Pharmaceuticals (NASDAQ: CURX) stands at this promising intersection. As a US-based, preclinical-stage biopharmaceutical company specializing in botanical drug development, Curanex is pioneering new possibilities for addressing a range of complex diseases.
Founded in 2018 and headquartered in Jericho, New York, Curanex achieved a significant milestone with its successful initial public offering on the NASDAQ stock market on August 26, 2025. This listing marks a pivotal new chapter, providing enhanced resources and visibility to advance its ambitious research and development pipeline.

The contemporary healthcare environment is witnessing a growing patient and scientific interest in naturally-derived treatment options. Botanical therapies, with their complex compositions and historical use, have emerged as a significant area of research focus. Curanex Pharmaceuticals is strategically positioned to leverage this trend. The company is dedicated to developing scientifically-validated botanical therapeutics for various conditions, effectively bridging the wisdom of traditional medicine with the rigor of modern biotechnology.
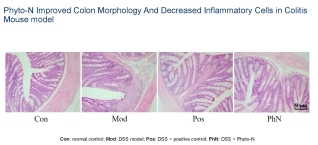
The company's leading drug candidate, Phyto-N, is a proprietary, multi-component botanical extract. Meticulously researched for its specific chemical profile and pharmacological activity, Phyto-N is engineered to possess enhanced anti-inflammatory properties. Its active constituents are derived from a botanical with a well-documented history of use within Traditional Chinese Medicine (TCM), a system boasting a rich repository of empirical clinical experience accumulated over centuries. Curanex's team of scientists uses this established foundation as a starting point, applying advanced modern biotechnological methods to meticulously characterize, standardize, and optimize the extract. This rigorous approach aims to develop a refined therapeutic agent that offers improved efficacy, consistency, and safety profiles compared to traditional herbal preparations.
Aligned with its strategic roadmap, Curanex Pharmaceuticals is on track to submit an Investigational New Drug (IND) application to the U.S. Food and Drug Administration (FDA) in 2025. This critical regulatory step will initiate the formal clinical trial process for Phyto-N, representing not just a major corporate milestone but also a significant advancement for the broader field of botanical medicine, moving it further into the mainstream of modern evidence-based therapeutics.
The therapeutic potential of Phyto-N is slated for exploration across multiple disease areas with significant unmet medical needs. The company's research agenda includes planned investigations for conditions such as atopic dermatitis, diabetes, non-alcoholic fatty liver disease (NAFLD), gout, and acne. These ailments collectively impact the health and quality of life of millions of patients globally. Curanex's R&D efforts hold the promise of delivering new, potentially safer, and more natural treatment alternatives for these widespread health challenges.
The path from preclinical discovery to an approved medicine is inherently long, complex, and capital-intensive. The biopharmaceutical journey is fraught with challenges, including the need to ensure batch-to-batch consistency of complex natural products, demonstrate unequivocal efficacy through controlled trials, and establish a comprehensive safety profile. Potential hurdles such as variable potency, unforeseen toxicology, or insufficient clinical response are inherent risks in any drug development program.
Curanex Pharmaceuticals, however, is equipped to navigate these challenges confidently. The company's strength lies in its specialized scientific personnel, who possess deep expertise in both phytochemistry and modern pharmacology. This team is supported by sophisticated technological platforms that facilitate the precise standardization of botanical ingredients and the detailed analysis of their biological mechanisms. The company's core competency is its profound understanding of the unique considerations involved in developing botanical drugs, differentiating its approach from conventional small-molecule or biologic development.
The company is committed to allocating substantial resources towards its R&D initiatives, continuously refining the pharmacological properties of Phyto-N. Through stringent experimental designs, robust data analysis, and continuous monitoring of key efficacy and safety indicators, Curanex aims to methodically de-risk the development process and make data-driven decisions to steer its research direction effectively.
As a biopharmaceutical enterprise that harmoniously blends innovative scientific principles with the foundational knowledge of traditional medicine, Curanex Pharmaceuticals is making deliberate and steady progress in the specialized field of botanical therapy. With its promising lead candidate and a clear strategic vision, Curanex is well-positioned to contribute meaningfully to the future of healthcare. As its research programs advance, the company has the potential to deliver groundbreaking therapeutic options to patients worldwide, aiming to make a substantial and enduring impact on the biopharmaceutical industry.
Media Contact
Company Name: Curanex Pharmaceuticals Inc
Contact Person: Jun Lin
Email: Send Email
City: Jericho
Country: United States
Website: www.curanexpharma.com





