Pharmacokinetic profile of oral transmucosal delivery of TH104 was comparable to intravenous delivery of reference drug
Positive safety/tolerability achieved with TH104 reporting a mild side-effect profile
Planning for Phase 2 trial for moderate-to-severe chronic pruritus in primary biliary cholangitis (PBC) is ongoing and on track to initiate in 2024
BRIDGEWATER, NJ / ACCESSWIRE / June 10, 2024 / Tharimmune, Inc. (Nasdaq:THAR) ("Tharimmune" or the "Company"), a clinical-stage biotechnology company developing a portfolio of therapeutic candidates for inflammation and immunology, reports positive results from its Phase 1 clinical trial with TH104, a proprietary transmucosal buccal film embedded with the approved, active compound nalmefene that easily adheres inside of the mouth on the cheek and biodegrades within minutes. Results in healthy subjects demonstrated consistent pharmacokinetic (PK) profiles across buccal and intravenous routes of administration with a comparable safety and tolerability profile between routes of administration.
"We are pleased with the results of our Phase 1 trial of TH104, and our expectations for a predictable kinetic profile while demonstrating excellent safety and tolerability by transmucosal administration have been met. With these results, we look forward to advancing TH104 to a Phase 2 trial this year," said Randy Milby, Chief Executive Officer of Tharimmune. "We would like to thank the volunteers for participating in this trial where the data generated are a critical step forward to continue our momentum and remain on track to advance the development of this important program."
TH104, the Company's lead clinical asset, is designed to avoid the liver's first-pass metabolism of oral formulations and, as such, may be a preferred product candidate for liver-related and other pruritogenic inflammatory conditions. The molecule has a dual mechanism of action that affects both the mu (μ-) and kappa opioid receptors. These extensively studied receptors, when stimulated and/or inhibited by the body's endogenous ligands, have been implicated in human itch circuitry for certain conditions, specifically cholestatic liver conditions.
This Phase 1 trial is a single-dose, single-center, open-label, randomized 2-way crossover study comparing 16mg of TH104 with 1mg intravenous nalmefene administered under fasting conditions, with a 7-day washout period between doses. Twenty healthy subjects were enrolled to complete both doses of the crossover design. All 20 subjects completed TH104 buccal dosing, while 19 of 20 subjects also completed the intravenous dosing. The primary objective was to evaluate the absolute bioavailability of TH104, as well as to assess safety and tolerability.
Findings from the study indicate that the primary endpoint of the absolute bioavailability (F) of TH104, or fraction (or percentage) of the administered dose absorbed into the systemic circulation compared to an equivalent intravenous dose of nalmefene, was 0.459 (45.9%). The median time to maximum concentration (Cmax) of TH104 was 2.0 hours, with rising concentrations beginning within minutes of dosing. The mean half-life (T1/2) as measured in the blood of subjects was 14 hours after a single buccal administration of TH104, compared to 9 hours for the 1mg intravenous dose of nalmefene.
These data are consistent and within range of published findings of population PK data of nine Phase 1 studies of 243 subjects with extensive blood sampling.1. Furthermore, in the same analysis, receptor occupancy of oral dosing of nalmefene using a robust PK model for nalmefene was developed where a single 20mg dose showed μ-opioid receptor occupancy was simulated to be within or above 60-90% for up to 22-24 hours.
The Company believes PK results from this Phase 1 trial show proportional kinetics consistent with published findings of oral and intravenous formulations of nalmefene including F, Cmax, T1/2 and potential receptor occupancy time, suggest TH104 could be developed for once-daily dosing in a target population of moderate-to-severe chronic pruritus in PBC patients.
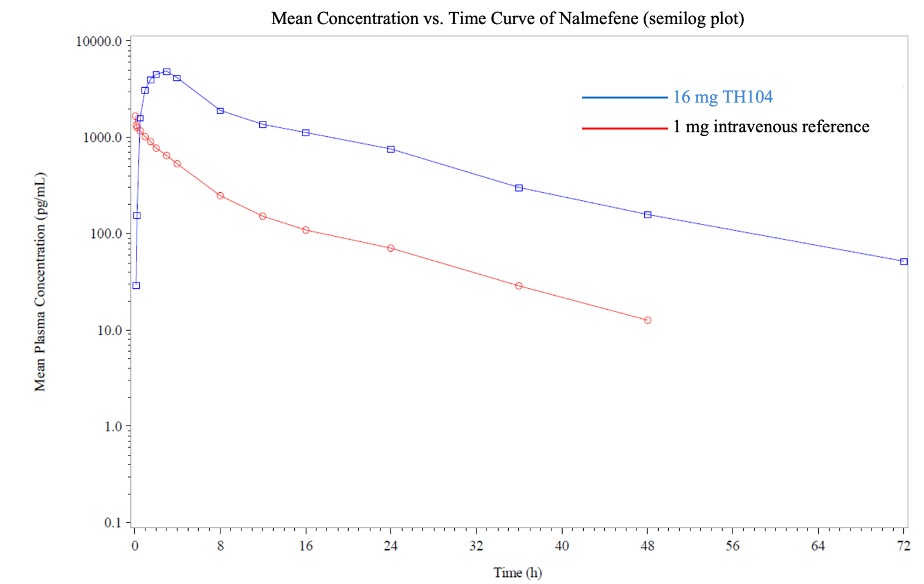
The Phase 1 trial also demonstrated that a 16mg dose of TH104 had a comparable safety and tolerability profile to the FDA-approved 1mg dose of nalmefene intravenous formulation. Treatment emergent adverse events (TEAEs) were reported in 8 subjects (40.0%) in the TH104 group and 7 subjects (36.8%) in the intravenous group. All reported TEAEs were considered mild in severity. The most frequently reported TEAE for both TH104 and intravenous treatments was dizziness (4 subjects in the TH104 group; 7 subjects in the intravenous group). TEAEs reported in at least 2 subjects in any treatment group were nausea (3 subjects in each group) and somnolence (3 subjects in each group).
There were no serious adverse events reported during this study. No subjects discontinued the study due to adverse events. No subjects exhibited abnormal results for the visual examinations of the buccal mucosa pre- or post-dosing with TH104 buccal film.
The Company continues to have discussions with the FDA regarding its Phase 2 program and intends to initiate a clinical trial with TH104 in moderate-to-severe chronic pruritus in PBC patients in 2024.
About TH104
TH104 is embedded with nalmefene onto a proprietary transmucosal buccal film which easily adheres to the inside of the mouth. This endows TH104 with key features making it an ideal product candidate for multiple liver-related and other pruritogenic inflammatory conditions. The molecule has a dual mechanism of action affecting both the µ-opioid and kappa opioid receptors. These opioid receptors when stimulated and/or inhibited by the body's natural ligands have been known to be involved in the body's itch circuitry.
About Tharimmune
Tharimmune, Inc. is a clinical-stage biotechnology company developing a portfolio of therapeutic candidates for inflammation and immunology. The Company's lead clinical-stage asset, TH104 is known to suppress chronic, debilitating pruritus or "uncontrollable itching" in PBC, a rare and orphan liver disease with no known cure. The Company's early-stage immunology pipeline includes novel multi-specific antibodies targeting unique epitopes with novel mechanisms of action against well-known, validated targets in multiple solid tumors, including PD-1, HER2 and HER3. Tharimmune has a license agreement with OmniAb, Inc. to access the company's antibody discovery technology platform against these and other specified targets. For more information please visit: www.tharimmune.com.
Forward Looking Statements
Certain statements in this press release are forward-looking within the meaning of the Private Securities Litigation Reform Act of 1995. All statements, other than statements of historical facts, contained in this press release, including statements regarding Tharimmune's strategy, future operations, future financial position, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words "anticipate," "believe," "continue," "could," "depends," "estimate," "expect," "intend," "may," "ongoing," "plan," "potential," "predict," "project," "target," "should," "will," "would," and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. The Company may not actually achieve the plans, intentions, or expectations disclosed in these forward-looking statements, and you should not place undue reliance on these forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements. Factors that may cause such differences, include, but are not limited to, those discussed under Risk Factors set forth in our Annual Report on Form 10-K for the year ended December 31, 2023 and other periodic reports filed by the Company from time to time with the Securities and Exchange Commission. In addition, the forward-looking statements included in this press release represent the Company's views as of the date of this release. Subsequent events and developments may cause the Company's views to change; however, the Company does not undertake and specifically disclaims any obligation to update or revise any forward-looking statements to reflect new information, future events or circumstances or to reflect the occurrences of unanticipated events, except as may be required by applicable law. These forward-looking statements should not be relied upon as representing the Company's views as of any date subsequent to the date of this release.
References
Kyhl, L. B., Shen, L., Faerch, K. U., Soegaard, B., Larsen, F., & Areberg, J. (2016). Population pharmacokinetics of nalmefene in healthy subjects in relation to μ-opioid receptor occupancy. British Journal of Clinical Pharmacology, 81(2), 290-300.
Contacts:
Tharimmune, Inc.
ir@tharimmune.com
LHA Investor Relations
Tirth T. Patel
tpatel@lhai.com
212-201-6614
SOURCE: Tharimmune, Inc.
View the original press release on accesswire.com





